Ciwon daji na huhu mara karami (NSCLC) yana da kusan kashi 80% -85% na adadin cutar kansar huhu, kuma aikin tiyata shine hanya mafi inganci don maganin radical na farkon NSCLC. Duk da haka, tare da raguwar 15% kawai a sake dawowa da kuma 5% ingantawa a cikin shekaru 5 na rayuwa bayan aikin chemotherapy, akwai wata babbar bukata ta asibiti.
Perioperative immunotherapy ga NSCLC wani sabon wurin bincike ne a cikin 'yan shekarun nan, kuma sakamakon da yawa na lokaci na 3 da aka sarrafa bazuwar gwaji sun kafa muhimmiyar matsayi na immunotherapy na perioperative.
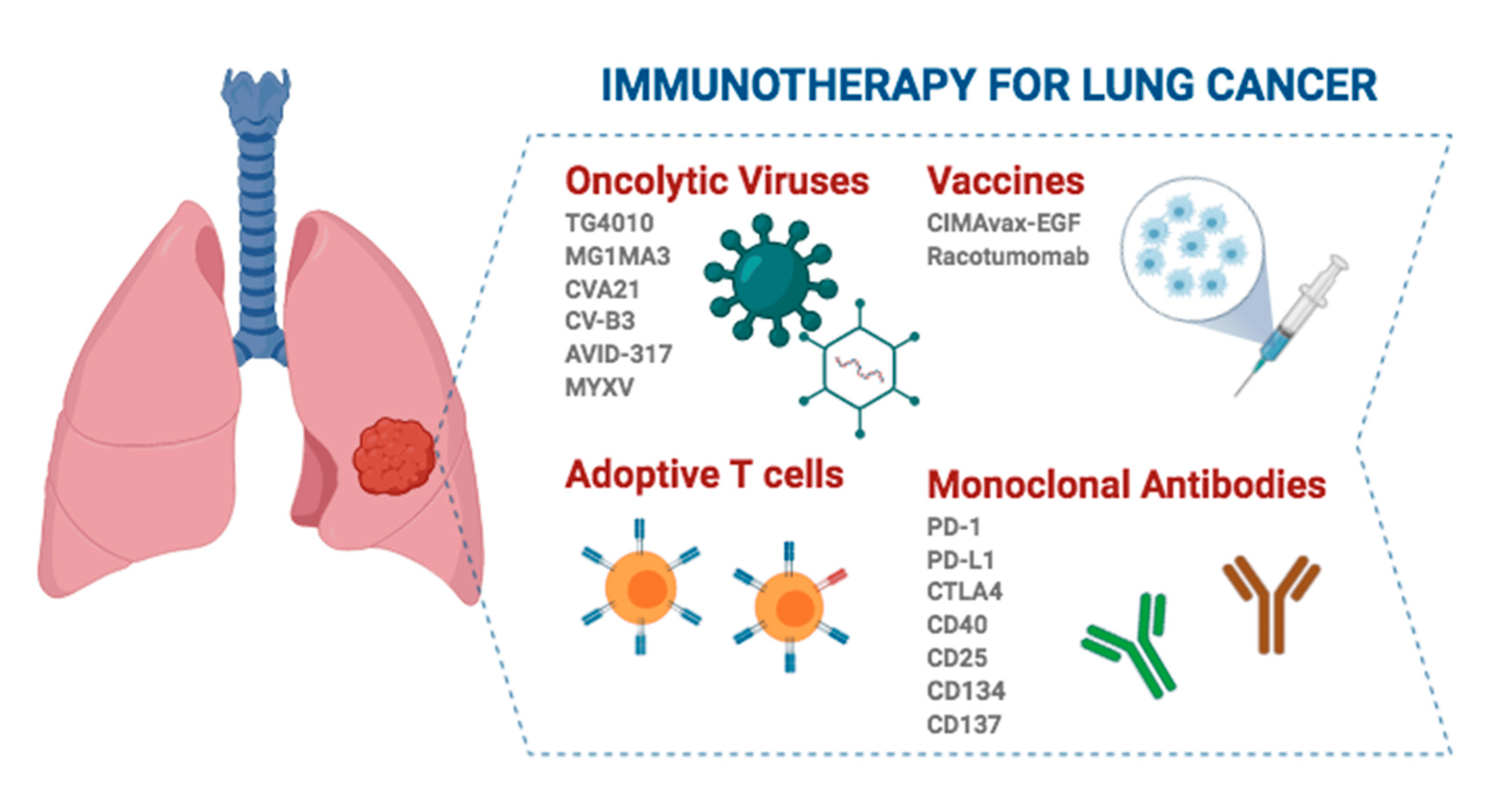
Immunotherapy ga marasa lafiya masu fama da ciwon huhu na huhu na farko (NSCLC) sun sami babban ci gaba a cikin 'yan shekarun nan, kuma wannan dabarun magani ba wai kawai ya kara rayuwar marasa lafiya ba, har ma yana inganta yanayin rayuwa, yana samar da ingantaccen kari ga aikin tiyata na gargajiya.
Dangane da lokacin da ake gudanar da rigakafi, akwai manyan alamu guda uku na immunotherapy a cikin kula da matakin farko na NSCLC:
1. Neoadjuvant immunotherapy kadai: Ana yin rigakafi kafin tiyata don rage girman ƙwayar cutar da kuma rage haɗarin sake dawowa. Binciken CheckMate 816 [1] ya nuna cewa immunotherapy haɗe tare da chemotherapy ya inganta rayuwa mara kyau (EFS) a cikin lokacin neoadjuvant idan aka kwatanta da chemotherapy kadai. Bugu da ƙari, immunotherapy neoadjuvant kuma zai iya rage yawan maimaitawa yayin da yake inganta ƙimar cikakkiyar amsawar cututtuka (pCR) na marasa lafiya, don haka rage yiwuwar sake dawowa bayan aiki.
2. Perioperative immunotherapy (neoadjuvant + adjuvant): A cikin wannan yanayin, ana gudanar da immunotherapy kafin da kuma bayan tiyata don haɓaka tasirin antitumor da kuma ƙara cire ƙananan raunuka bayan tiyata. Maƙasudin maƙasudin wannan samfurin jiyya shine don inganta rayuwa na dogon lokaci da kuma maganin adadin marasa lafiya da ciwon daji ta hanyar hada maganin rigakafi a cikin matakan neoadjuvant (pre-operative) da adjuvant (bayan aiki). Maɓalli na 671 shine wakilin wannan ƙirar [2]. A matsayin kawai gwajin sarrafawa bazuwar (RCT) tare da tabbataccen EFS da ƙarshen ƙarshen OS, ya kimanta ingancin palizumab tare da chemotherapy a cikin matakan da za a iya jurewa Ⅱ, ⅢA, da ⅢB (N2) marasa lafiya NSCLC. Idan aka kwatanta da chemotherapy kadai, pembrolizumab hade tare da chemotherapy ya tsawaita matsakaicin EFS ta shekaru 2.5 kuma ya rage haɗarin ci gaba da cututtuka, sake dawowa, ko mutuwa ta 41%; KEYNOTE-671 kuma shine binciken farko na rigakafi don nuna fa'idar rayuwa gabaɗaya (OS) a cikin NSCLC mai daidaitawa, tare da raguwar 28% cikin haɗarin mutuwa (HR, 0.72), wani ci gaba a cikin neoadjuvant da adjuvant immunotherapy don aiki na farkon matakin NSCLC.
3. Adjuvant immunotherapy kadai: A cikin wannan yanayin, marasa lafiya ba su sami maganin miyagun ƙwayoyi ba kafin a yi musu tiyata, kuma an yi amfani da magungunan rigakafi bayan tiyata don hana sake dawowa na ciwace-ciwacen ƙwayar cuta, wanda ya dace da marasa lafiya tare da haɗari mai yawa. Nazarin IMpower010 ya kimanta ingancin adjuvant attilizumab bayan tiyata tare da ingantacciyar hanyar tallafi a cikin marasa lafiya tare da cikakkiyar matakin IB zuwa IIIA (AJCC 7th edition) NSCLC [3]. Sakamakon ya nuna cewa haɗin gwiwa tare da attilizumab yana daɗaɗawa sosai ga rayuwa mara cuta (DFS) a cikin PD-L1 tabbatacce marasa lafiya a mataki ⅱ zuwa ⅢA. Bugu da ƙari, binciken KEYNOTE-091 / PEARLS ya kimanta tasirin pembrolizumab a matsayin maganin haɗin gwiwa a cikin marasa lafiya marasa lafiya tare da mataki na IB zuwa IIIA NSCLC [4]. Pabolizumab ya kasance mai tsayi sosai a cikin yawan jama'a (HR, 0.76), tare da tsaka-tsakin DFS na watanni 53.6 a cikin ƙungiyar Pabolizumab da watanni 42 a cikin ƙungiyar placebo. A cikin rukuni na marasa lafiya tare da PD-L1 tumor proportion score (TPS) ≥50%, ko da yake DFS ya tsawaita a cikin rukunin Pabolizumab, bambancin da ke tsakanin ƙungiyoyin biyu ba shi da mahimmanci saboda ƙananan ƙananan samfurin, kuma ana buƙatar ƙarin bibiya don tabbatarwa.
Dangane da ko an haɗa immunotherapy tare da wasu magunguna ko matakan warkewa da yanayin haɗin gwiwa, shirin na immunotherapy neoadjuvant immunotherapy da adjuvant immunotherapy za a iya raba zuwa manyan hanyoyi uku masu zuwa:
1. Immunotherapy guda ɗaya: Irin wannan maganin ya haɗa da nazarin irin su LCMC3 [5], IMpower010 [3], KEYNOTE-091 / PEARLS [4], BR.31 [6], da ANVIL [7], wanda aka kwatanta da yin amfani da magungunan rigakafi guda ɗaya a matsayin (sabon) maganin adjuvant.
2. Haɗin immunotherapy da chemotherapy: Irin waɗannan karatun sun haɗa da KEYNOTE-671 [2], CheckMate 77T [8], AEGEAN [9], RATIONALE-315 [10], Neotorch [11], da IMpower030 [12]. Wadannan binciken sun kalli tasirin hada immunotherapy da chemotherapy a cikin lokacin aiki.
3. Haɗuwa da immunotherapy tare da sauran hanyoyin magani: (1) Haɗuwa tare da sauran magungunan rigakafi: Misali, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) an haɗa shi a cikin gwajin NEOSTAR [13], lymphocyte activation gene 3 (LAG-3) antibody an hade a cikin NEO-Predict-Lung, tsarin rigakafi da kuma TIM a cikin tsarin IM. Gwajin SKYSCRAPER 15 Nazarin irin su TIGIT antibody hade [15] sun inganta tasirin anti-tumor ta hanyar haɗin magungunan rigakafi. (2) Haɗe tare da radiotherapy: alal misali, duvaliumab hade tare da stereotactic radiotherapy (SBRT) an tsara shi don haɓaka tasirin warkewa na farkon NSCLC [16]; (3) Haɗuwa da magungunan anti-angiogenic: Misali, binciken EAST ENERGY [17] ya bincika tasirin haɗin gwiwa na ramumab tare da immunotherapy. Binciken hanyoyin rigakafin rigakafi da yawa ya nuna cewa tsarin aikace-aikacen immunotherapy a cikin lokacin aiki ba a fahimta sosai ba. Kodayake immunotherapy kadai ya nuna sakamako mai kyau a cikin jiyya na perioperative, ta hanyar hada chemotherapy, radiation therapy, antiangiogenic therapy, da sauran masu hanawa na rigakafi irin su CTLA-4, LAG-3, da TIGIT, masu bincike suna fatan kara inganta ingantaccen maganin rigakafi.
Har yanzu babu wani ƙarshe game da mafi kyawun yanayin rigakafi don aiki na farkon NSCLC, musamman ma idan aka kwatanta da aikin immunotherapy idan aka kwatanta da neoadjuvant immunotherapy kadai, kuma ko ƙarin maganin rigakafi na iya haifar da ƙarin sakamako masu mahimmanci, har yanzu akwai ƙarancin sakamako mai kamanta kai tsaye.
Forde et al. yayi amfani da ƙididdigar ƙima mai ma'auni mai ma'auni don daidaita tasirin gwaje-gwajen da aka sarrafa bazuwar, da daidaita ƙayyadaddun ƙayyadaddun alƙaluma da halaye na cuta a tsakanin yawan binciken daban-daban don rage rikicewar waɗannan abubuwan, yana mai da sakamakon CheckMate 816 [1] da CheckMate 77T [8] mafi kamanta. Lokacin bin tsaka-tsaki shine watanni 29.5 (CheckMate 816) da watanni 33.3 (CheckMate 77T), bi da bi, yana ba da isasshen lokacin bibiya don kiyaye EFS da sauran mahimman matakan inganci.
A cikin nazarin ma'auni, HR na EFS shine 0.61 (95% CI, 0.39 zuwa 0.97), yana ba da shawarar 39% ƙananan haɗari na sake dawowa ko mutuwa a cikin nabuliumab na haɗin gwiwa na ƙungiyar chemotherapy (CheckMate 77T yanayin) idan aka kwatanta da neoadjuvant nabuliumab hade chemotetherapy (1ck). Ƙungiyar nebuliyuzumab da ke aiki tare da ƙungiyar chemotherapy sun nuna fa'ida mai sauƙi a cikin duk marasa lafiya a matakin asali, kuma tasirin ya fi bayyana a cikin marasa lafiya da ke da ƙasa da 1% ƙwayar ƙwayar cuta ta PD-L1 (raguwa 49% cikin haɗarin sake dawowa ko mutuwa). Bugu da ƙari, ga marasa lafiya waɗanda suka kasa samun pCR, ƙungiyar naburiumab na haɗin gwiwar chemotherapy sun nuna babban fa'ida na EFS (35% raguwa a cikin haɗarin sake dawowa ko mutuwa) fiye da neoadjuvant nabuliumab hade ƙungiyar chemotherapy. Wadannan sakamakon sun nuna cewa samfurin immunotherapy na perioperative yana da amfani fiye da samfurin immunotherapy neoadjuvant kadai, musamman ma a cikin marasa lafiya tare da ƙananan maganganun PD-L1 da ragowar tumor bayan jiyya na farko.
Koyaya, wasu kwatancen kai tsaye (irin su meta-bincike) ba su nuna wani muhimmin bambanci a cikin rayuwa tsakanin neoadjuvant immunotherapy da perioperative immunotherapy [18]. Wani bincike-bincike bisa bayanan mai haƙuri na mutum ya gano cewa aikin immunotherapy da neoadjuvant immunotherapy suna da sakamako iri ɗaya akan EFS a cikin ƙungiyoyin pCR da waɗanda ba na PCR ba a cikin marasa lafiya tare da farkon matakin NSCLC [19]. Bugu da ƙari, gudunmawar lokaci na immunotherapy adjuvant, musamman bayan da marasa lafiya suka sami pCR, ya kasance wani abu mai rikitarwa a cikin asibitin.
Kwanan nan, Hukumar Kula da Abinci da Magunguna ta Amurka (FDA) Kwamitin Ba da Shawarwari kan Magungunan Oncology ya tattauna wannan batu, yana mai jaddada cewa takamaiman rawar da ke tattare da rigakafin rigakafi har yanzu ba a san shi ba [20]. An tattauna cewa: (1) Yana da wuya a bambance tasirin kowane mataki na jiyya: saboda tsarin aikin tiyata ya ƙunshi nau'i biyu, neoadjuvant da adjuvant, yana da wuya a ƙayyade gudunmawar mutum ɗaya na kowane lokaci zuwa ga gaba ɗaya, yana da wuya a tantance wane lokaci ya fi mahimmanci, ko kuma ana buƙatar aiwatar da matakai biyu a lokaci guda; (2) Yiwuwar haɓakawa: idan immunotherapy ya shiga cikin nau'ikan jiyya guda biyu, yana iya haifar da marasa lafiya su sami ƙarin magani da haɓaka haɗarin sakamako masu illa; (3) Ƙarfafa nauyin jiyya: Ƙarin jiyya a cikin lokacin jiyya na adjuvant zai iya haifar da nauyin jiyya mafi girma ga marasa lafiya, musamman ma idan akwai rashin tabbas game da gudunmawarsa ga ingancin gabaɗaya. Dangane da muhawarar da ke sama, domin a zayyana tabbataccen ƙarshe, ana buƙatar ƙarin ƙwaƙƙwaran ƙirƙira gwaje-gwajen sarrafawa don ƙarin tabbaci a nan gaba.
Lokacin aikawa: Dec-07-2024